What is in this leaflet
Read this leaflet carefully before taking your medicine.
This leaflet answers some common questions about ziprasidone. It does not contain all the available information. It does not take the place of talking to your doctor or pharmacist.
The information in this leaflet was last updated on the date listed on the last page. More recent information on this medicine may be available.
Ask your doctor or pharmacist:
- if there is anything you do not understand in this leaflet,
- if you are worried about taking your medicine, or
- to obtain the most up-to-date information.
All medicines have risks and benefits. Your doctor has weighed the risks of you using this medicine against the benefits they expect it will have for you.
Pharmaceutical companies cannot give you medical advice or an individual diagnosis.
Keep this leaflet with your medicine. You may want to read it again.
What this medicine is used for
The name of your medicine is ZIPROX. It contains the active ingredient ziprasidone (as ziprasidone hydrochloride).
It is used to treat schizophrenia and bipolar disorder.
Ziprasidone is available only with a doctor's prescription.
There is no evidence that this medicine is addictive.
Schizophrenia
Schizophrenia is a mental illness. It varies from person to person, but can involve:
- hallucinations: the person sees, hears, feels, smells or tastes something that is not actually there; most commonly a person may hear voices
- delusions: a delusion is a false belief held by a person which is not held by others of the same cultural background
- disturbed or disorganised thinking
- poor memory and concentration
- loss of emotion and expression
- loss of motivation and energy
- difficulty interacting with others, leading to social isolation.
Bipolar disorder
Bipolar disorder is a mental illness where a person cycles through:
- 'manic' phases - with symptoms such as over-activity, irritability, elation and limited need for sleep
- 'depressive' phases - with symptoms such as depressed mood, anxiety, difficulty making decisions, difficulty concentrating and hopelessness.
How it works
Ziprasidone belongs to a group of medicines called atypical antipsychotics/neuroleptics.
Schizophrenia
Researchers do not know exactly what causes schizophrenia, but they do know that many people with it have high levels of some brain chemicals - including dopamine and serotonin.
Ziprasidone is thought to work by helping to correct the imbalance of these chemicals, in turn, reducing the symptoms of schizophrenia.
Research has found ziprasidone can help reduce:
- hallucinations
- delusions
- confused thoughts
- social withdrawal
- lack of motivation.
Ziprasidone does not cure schizophrenia, but it can help manage the symptoms and help prevent further episodes.
Taking antipsychotic/neuroleptic medicine like ziprasidone can also allow you to try psychological therapies when recommended by your doctor. These may further help you manage your schizophrenia.
Bipolar disorder
Research has shown that there is a chemical imbalance in the brain in patients with bipolar disorder.
Ziprasidone does not cure bipolar disorder. It is used as a short-term treatment for the manic phases. It is not used to treat the depressive phases of bipolar disorder.
Controlling the manic phase of bipolar disorder with medicine can also allow you to try psychological therapies when recommended by your doctor.
Elderly patients
Ziprasidone is not recommended for the treatment of elderly patients with dementia-related psychosis.
It should be used with caution in elderly patients with risk factors for stroke.
Use in children
Ziprasidone is not recommended for children under 18 years of age as there is not enough information on its effects in this age group.
Before you take this medicine
When you must not take it
Do not take this medicine if:
-
You have or have had any of the following:
- a recent heart attack
- heart failure that is not well- controlled
- a condition that may lengthen your heart rhythm
- a condition requiring medication to control (lengthen or shorten) your heart rhythm. - You are taking any other medications known to lengthen your heart rhythm.
-
You are hypersensitive to, or have had an allergic reaction to, ziprasidone or any of the ingredients listed at the end of this leaflet.
Symptoms of an allergic reaction may include cough, shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue, throat or other parts of the body, rash, itching or hives on the skin; fainting or hayfever- like symptoms
If you think you arehaving an allergic reaction, do not take any more of the medicine and contact your doctor immediately or go to the Accident and Emergency department at the nearest hospital. - The expiry date (EXP) printed on the pack has passed.
- The packaging is torn, shows signs of tampering or it does not look quite right.
Before you start to take it
Before you start taking this medicine, tell your doctor if:
- You have allergies to:
- any other medicines
- any other substances, such as foods, preservatives or dyes.
- You have or have had any medical conditions, especially the following:
- irregular heart rate
- any heart or blood vessel problems
- low blood levels of potassium or magnesium
- a condition that may give you low blood pressure
- seizures (fits)
- liver problems
- blood sugar level problems, e.g. diabetes
- you are 65 years of age or over and have a condition known as 'dementia-related psychosis'
- Neuroleptic Malignant Syndrome (NMS) - symptoms include sudden fever, fast breathing, blood pressure changes, sweating, confusion, muscle stiffness and drowsiness or sleepiness
- Tardive Dyskinesia (TD) – symptoms include unusual movements (mainly of the face and tongue), or uncontrollable twitching or jerking of the arms and legs.
- You are currently pregnant or you plan to become pregnant.
Like most atypical antipsychotic/ neuroleptic medicines, ziprasidone is not recommended for use during pregnancy.
Do not take this medicine whilst pregnant until you and your doctor have discussed the risks and benefits involved.
For women of child-bearing age an appropriate method of contraception is recommended
- You are currently breast-feeding or you plan to breast-feed.
Do not take this medicine whilst breast-feeding until you and your doctor have discussed the risks and benefits involved. It is not known if it passes into breast- milk
- You are planning to have surgery or an anaesthetic.
- You are currently receiving or are planning to receive dental treatment.
- You are taking or are planning to take any other medicines. This includes vitamins and supplements that are available from your pharmacy, supermarket or health food shop.
Some medicines may interact with ziprasidone. These include:
- lithium, a medicine used to treat bipolar disorders
- medicines which act on the central nervous system (CNS), such as triptans, antipsychotics and antidepressants
- alcohol
- medicines used to control (lengthen or shorten) heart rhythm
- ketoconazole, a medicine used to treat fungal infections
- carbamazepine, a medicine used to treat epilepsy.
Your doctor and pharmacist have more information on medicines to be careful with or avoid while taking ziprasidone.
If you are taking any of these you may need a different dose or you may need to take different medicines.
Other medicines not listed above may also interact with ziprasidone.
How to take this medicine
Follow carefully all directions given to you by your doctor. Their instructions may be different to the information in this leaflet.
How much to take
Your doctor will tell you how much of this medicine you should take.
This will depend on your condition and whether you are taking any other medicines.
Do not stop taking your medicine or change your dosage without first checking with your doctor.
Schizophrenia
The usual starting dose is one 40 mg capsule taken twice daily with food.
Your doctor may increase your dose up to one 80 mg capsule twice daily with food. To help you increase your dose, your doctor may give you a specially designed pack, known as a 'Titration Pack'.
Bipolar disorder
The usual starting dose is one 40 mg capsule twice daily with food.
Your doctor may adjust your dose up to one 80 mg capsule twice daily with food.
How to take it
Swallow the capsule(s) whole with a glass of water.
When to take it
Take your capsule(s) with food, so in the morning with breakfast and in the evening with your evening meal.
You need to take ziprasidone with food because it helps your body absorb the medicine better. If you do not take with food, the medicine may have less effect.
Take this medicine at the same times each day. Taking it at the same times each day will have the best effect and will also help you remember when to take it.
How long to take it for
Continue taking your medicine for as long as your doctor tells you.
Make sure you have enough to last over weekends and holidays.
If you forget to take it
If it is almost time to take your next dose, skip the missed dose and take your next dose at the usual time.
Otherwise take it as soon as you remember and then go back to taking your medicine as you would normally.
Do not take a double dose to make up for missed doses. This may increase the chance of you experiencing side effects.
If you have trouble remembering to take your medicine, ask your pharmacist for some hints to help you remember.
If you take too much (overdose)
If you think that you or anyone else may have taken too much of this medicine, immediately telephone your doctor or the Poisons Information Centre (Tel: 13 11 26 in Australia) for advice. Alternatively go to the Accident and Emergency Department at your nearest hospital.
Do this even if there are no signs of discomfort or poisoning. You may need urgent medical attention.
If you take too much ziprasidone, you may feel drowsy and show signs of tremor and uncontrollable movements of the tongue, jaw, arms and legs.
While you are taking this medicine
Things you must do
Tell your doctor that you are taking this medicine if:
- you are about to be started on any new medicine
- you are pregnant or are planning to become pregnant
- you are breast-feeding or are planning to breast-feed
- you are about to have any blood tests
- you are going to have surgery or an anaesthetic or are going into hospital.
Your doctor may occasionally do tests to make sure the medicine is working and to prevent side effects. Go to your doctor regularly for a check-up.
Tell any other doctors, dentists and pharmacists who are treating you that you take this medicine.
Things you must not do
Do not:
- take your medicine to treat any other condition unless your doctor or pharmacist tells you to
- give this medicine to anyone else, even if their symptoms seem similar to yours
- stop taking your medicine, or change the dosage, without first checking with your doctor.
Things to be careful of
Be careful when driving or operating machinery until you know how this medicine affects you.
If you feel drowsy or sleepy while taking ziprasidone, do not drive or operate machinery, or do things that could be dangerous if you are not alert.
Be careful when drinking alcohol while taking ziprasidone. Combining ziprasidone and alcohol can make you more sleepy, dizzy or light-headed. Your doctor may suggest you avoid alcohol while you are being treated with ziprasidone.
Possible side effects
Tell your doctor or pharmacist as soon as possible if you do not feel well while you are taking this medicine or if you have any questions or concerns.
Do not be alarmed by the following lists of side effects. You may not experience any of them.
All medicines can have side effects.
Sometimes they are serious but most of the time they are not.
Tell your doctor if you notice any of the following.
This list includes the more common side effects:
- headache
- feeling sick (nausea)
- sleepiness
- difficulty sleeping
- dizziness on standing up, especially when getting up from a sitting or lying position
- dry mouth
- too much saliva
- indigestion
- constipation
- diarrhoea
- restlessness
- muscle stiffness
- blurred vision
- palpitations
- weakness or loss of strength
- drowsiness
- dizziness, blackouts or feeling faint
- anxiety or agitation.
- persistent painful erection of the penis or clitoris.
Tell your doctor immediately if you notice any of the following.
These may be serious side effects. You may need medical attention.
- any worm-like movements of the tongue
- any other uncontrolled movements of the tongue, mouth, cheeks or jaw
- any uncontrolled movements spreading to the arms and legs.
These are symptoms of a condition called Tardive Dyskinesia (TD).
If you experience any of the following, stop taking your medicine and contact your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
These are very serious side effects and can also occur after you stop taking ziprasidone. You may need urgent medical attention or hospitalisation.
- convulsions, fit or seizures
- trembling and shaking of the hands and fingers
- shuffling walk and stiffness of the arms and legs
- sudden uncontrollable muscle spasms in the eyes, head, neck and body
- symptoms of a condition called Neuroleptic Malignant Syndrome (NMS), which include: high fever, fast breathing, stiff muscles and confusion, drowsiness or sleepiness.
Tell your doctor if you notice anything else that is making you feel unwell. Other side effects not listed above may occur in some people.
Allergic reactions
If you think you are having an allergic reaction to ziprasidone, do not take any more of this medicine and tell your doctor immediately or go to the Accident and Emergency department at your nearest hospital.
Symptoms of an allergic reaction may include some or all of the following:
- cough, shortness of breath, wheezing or difficulty breathing.
- swelling of the face, lips, tongue, throat or other parts of the body
- rash, itching or hives on the skin
- fainting
- hayfever-like symptoms
Storage and disposal
Storage
Keep your medicine in its original packaging until it is time to take it.
If you take your medicine out of its original packaging it may not keep well.
Keep your medicine in a cool dry place where the temperature will stay below 25°C.
Do not store your medicine, or any other medicine, in the bathroom or near a sink. Do not leave it on a window sill or in the car. Heat and dampness can destroy some medicines.
Keep this medicine where children cannot reach it. A locked cupboard at least one-and- a-half metres above the ground is a good place to store medicines.
Disposal
If your doctor or pharmacist tells you to stop taking this medicine or it has passed its expiry date, your pharmacist can dispose of the remaining medicine safely.
Product description
What ZIPROX looks like
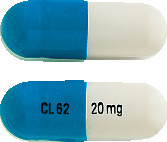
20 mg capsules: Blue/White size ‘4’ hard gelatin capsules imprinted in black ink with “CL62” on the cap and “20mg” on the body; filled with pale pink coloured powder.
40 mg capsules: Blue/Blue size ‘2’ hard gelatin capsules imprinted in black ink with “CL63” on the cap and “40mg” on the body; filled with pale pink coloured powder.
60 mg capsules: White/White size ‘1’ hard gelatin capsules imprinted in black ink with “CL64” on the cap and “60mg” on the body; filled with pale pink coloured powder.
80 mg capsules: Blue/White size ‘0’ hard gelatin capsules imprinted in black ink with “CL65” on the cap and “80mg” on the body; filled with pale pink coloured powder.
All strengths are available in blister packs of 60 capsules.
Ingredients
Each capsule contains ziprasidone hydrochloride as the active ingredient.
It also contains the following inactive ingredients:
- sodium starch glycollate (type B)
- macrogol 6000
- lactose
- sucrose
- ammonium chloride
- sodium lauryl sulfate
- gelatin
- titanium dioxide
- indigo carmine (20 mg, 40 mg and 80 mg capsules only)
- black printing ink
- purified water.
This medicine is gluten-free, tartrazine-free and free of other azo dyes.
Contains sugars as lactose, and sucrose.
May contains trace amounts of sulfites and phenylalanine.
Australian Registration Numbers
ZIPROX 20 mg: AUST R 201081.
ZIPROX 40 mg: AUST R 201082.
ZIPROX 60 mg: AUST R 201083.
ZIPROX 80 mg: AUST R 201084.
Sponsor
Arrotex Pharmaceuticals Pty Ltd
15 - 17 Chapel Street
Cremorne VIC 3121
This leaflet was revised in May 2023
Published by MIMS July 2023