MedicineInsight application process
MedicineInsight collects and uses data on the prescribing behaviour of general practitioners in Australia.
The independent Data Governance Committee provides advice to NPS MedicineWise on general data governance issues, and also reviews and, where appropriate, approves applications to access MedicineInsight data. The Committee is made up of consumer advocates, privacy and security experts, general practitioners and researchers.
The decision-making process of the Data Governance Committee takes into account study feasibility, research outputs, ethical appropriateness, benefits and public interest, secondary uses of the data as agreed by the data owners and potential risks with community perception.
Find out more about the members of the Data Governance Committee.
2020 Data Governance Committee - meeting dates and deadlines
| Data Governance Committee meeting dates | Data Governance Committee meeting dates |
|---|---|
| Wed, 10 February 2021 | 20 January 2021 |
| Wed, 07 April 2021 | 24 March 2021 |
| Wed, 09 June 2021 | 19 May 2021 |
| Wed, 11 August 2021 | 21 July 2021 |
| Wed, 13 October 2021 | 22 September 2021 |
| Wed, 08 December 2021 | 17 November 2021 |
How do I get access to MedicineInsight data or reports?
An overview of the process for accessing MedicineInsight data or reports is shown below. A member of the Client Relations team will work with you to facilitate access to the most appropriate MedicineInsight data products in a timely fashion.
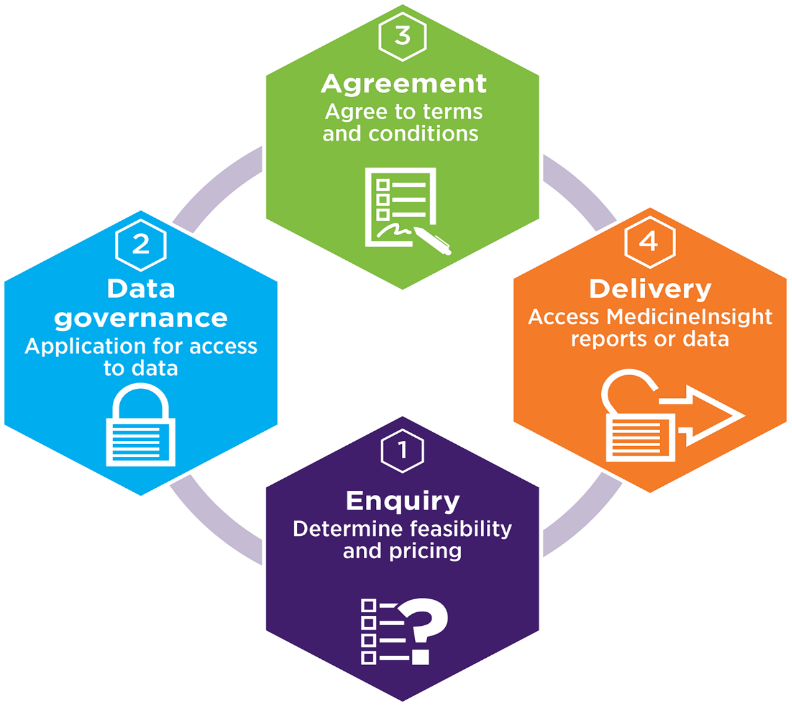
The first step to getting access to MedicineInsight data is to complete the Data Access Enquiry Form, available in the Research Kit. This form will help us understand your project and whether the data available in MedicineInsight can potentially answer your research questions.
For help completing the form, please email medicineinsight@nps.org.au or call us on 1300 721 726.
Once feasibility and price are determined and agreed, the next step is the formal governance approval process for access to MedicineInsight data.
How much will access to the MedicineInsight data cost?
The cost of access to MedicineInsight data or a report will vary depending on what you request. Once the Client Relations team has an understanding of your request and your budget, we will discuss the options available. We are also able to provide a formal proposal or quotation for submitting as part of an application for research funding.
Example uses of MedicineInsight data
MedicineInsight data is being used for a range of activities, some of which include:
- post-marketing surveillance of drugs prescribed for chronic obstructive pulmonary disease (COPD), diabetes and asthma, as well as antidepressants, anticoagulants, testosterone and antibiotics
- informing medicines policy, including a review of biological medicines used in general practice, monitoring the impact of changes to PBS restrictions for testosterone, and reviewing the use of antibiotics commonly used for respiratory tract infections
- supporting quality improvement in general practice by comparing practice activity with best practice guidelines, in clinical areas such as diabetes, stroke, COPD, depression and antibiotic use. This allows practice staff to reflect on current practice, identify areas for improvement and see where changes can be implemented
- primary care research, including evaluation of vaccination coverage, cardiovascular disease, chronic kidney disease, diabetes, pain, obesity and lung cancer.
