Antimicrobial use (AU) is a key driver of antimicrobial resistance (AMR) – the more we use antimicrobials, the more likely it is that resistance will develop.1 Appropriate use of antimicrobials can be life-saving, but inappropriate use needs to be minimised to prevent and contain AMR.
Informed by a range of high-quality data collections and analysis by the Australian Commission on Safety and Quality in Health Care, the first comprehensive surveillance report on AU and AMR in Australia is now available. The report includes important information for all prescribers, and the community.2
This article was prepared by the AURA Team at the Australian Commission on Safety and Quality in Health Care.


Key points
- High volumes of antimicrobials are being prescribed unnecessarily for colds and non-specific URTIs.
- A large proportion of patients with acute tonsillitis, acute or chronic sinusitis, acute otitis media or acute bronchitis are being inappropriately prescribed an antimicrobial.
- Many repeat prescriptions are being given when they are not needed.
- One in five antimicrobial prescriptions written in residential aged care facilities are for residents who have no signs or symptoms of infection according to international published criteria.3
Antimicrobial resistances and trends of concern include:
- 10.8% of Staphylococcus aureus infections in the community are methicillin-resistant S. aureus (MRSA)
- Escherichia coli resistant to all beta-lactams available in the community, including the third-generation cephalosporins
- resistance to macrolides (eg, roxithromycin), tetracyclines (eg, doxycycline) and trimethoprim–sulfamethoxazole (co-trimoxazole) in Streptococcus pneumoniae
- decreased susceptibility to ceftriaxone in Neisseria gonorrhoeae
- resistance to ciprofloxacin in Shigella species infections.2
Antimicrobial Use and Resistance in Australia (AURA)
Antimicrobial resistance (AMR) is a critical and immediate challenge to health systems in Australia and around the world.4, 5, 6
While AMR is to some extent a naturally occurring phenomenon,7 high and inappropriate antimicrobial use (AU) has accelerated increasing resistance worldwide.1, 2
The Australian Commission on Safety and Quality in Health Care (the Commission) has released a landmark report on AU and AMR in Australia.
AURA 2016: First Australian report on antimicrobial use and resistance in human health (AURA 2016) contains data on:2
- organisms deemed to be a priority for Australia
- the volume of AU
- the appropriateness of prescribing
- key emerging issues for AMR
- a comparison of Australia’s situation with other countries.
This article provides highlights from AURA 2016 for Australian hospitals, the community and residential aged care.
Key findings
Patterns of use
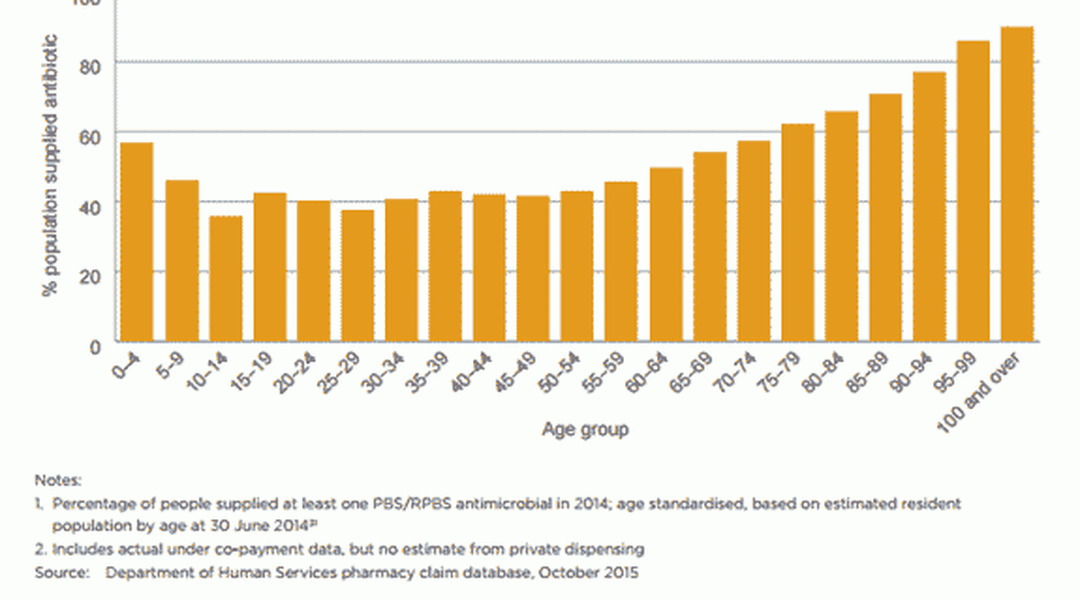
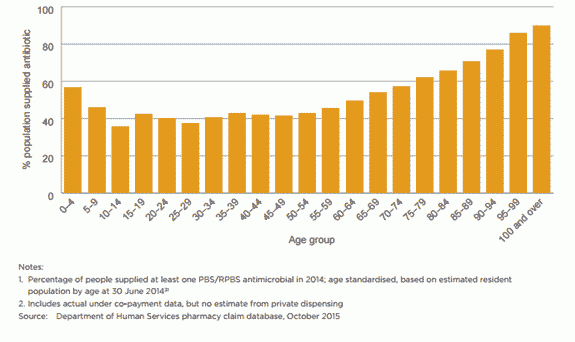
AU in the Australian community is higher than in other similar countries, with 46% of the population being dispensed at least one systemic antimicrobial prescription in 2014. Antimicrobial use is highest in children (0–9 years old) and older people (aged 65 and over).2
Different antimicrobial dispensing rates were seen across different states and territories, between major cities and other regions, and between different local areas. Apart from some local variation being attributable to higher infection rates in areas of lower socio-economic status, the primary reasons for this variation are unclear.
Penicillins are the most commonly prescribed antimicrobial group in the community, followed by cephalexin. Children are prescribed more extended-spectrum penicillins, and older people are prescribed more cephalosporins, macrolides and beta lactamase inhibitor combinations than other age groups.
Appropriateness of prescribing
In 2014 more than 50% of people with colds and other non-specific URTIs were prescribed an antimicrobial when it was was not recommended by national guidelines.2, 8
Some antimicrobials are prescribed more in winter, which suggests that they are potentially misused to treat viral illnesses such as colds and influenza. However, the national trend for inappropriate prescribing for URTIs is decreasing slowly, with systemic antimicrobials prescribed by GPs decreasing from 32.8% in 2011–12 to 29.0% in 2013–14.2 The European Surveillance of Antimicrobial Consumption (ESAC) report on disease-specific quality indicators suggests an acceptable range for this prescribing should be less than 20%.9
A large proportion of patients with acute tonsillitis, acute or chronic sinusitis (sinus inflammation), acute otitis media (middle ear infection) or acute bronchitis received an antimicrobial, despite antimicrobial treatment not being routine therapy in most cases.2, 9 A large proportion of antimicrobials prescribed were not those recommended by guidelines.10
Other reasons for inappropriate prescribing included incorrect antimicrobial choice, and incorrect duration of treatment. Many repeat prescriptions were also given when they were not needed.
In residential aged care facilities there is also some evidence of inappropriate use, with 11.3% of residents using antimicrobials but only 4.5% with a suspected or confirmed infection.
AMR in the community
Analysis of data has firmly established that there are a number of organisms in the Australia community of particular concern.2
Analysis shows that 10.8% of S. aureus infections in the community are MRSA. Community strains of MRSA now cause a significant number of infections in the community and are resulting in hospitalisation, with community-associated MRSA clones (including those in aged care facilities) now overtaking hospital-associated clones in hospital-onset staphylococcal sepsis.
E. coli is the most common cause of UTI and septicaemia. At present Australia has low rates of resistance to fluoroquinolones in E. coli compared with other countries, reflecting the restricted use of this antimicrobial class locally. By contrast, E. coli resistance to the beta-lactams available in the community, including amoxicillin, amoxicillin–clavulanate, cephalexin and the third-generation cephalosporins is increasing. Data show that cephalexin is being widely used for UTIs and skin and soft tissue infections, although it is not recommended as a first-line treatment for these indications.9
Macrolide, tetracycline and trimethoprim–sulfamethoxazole resistance in S. pneumoniae is now 20–30%, limiting second-line treatment options for bacterial lower respiratory tract infections in the community.
Due to its low prevalence, limited data on Shigella species are available. However, the presence of resistance to a key antimicrobial, ciprofloxacin, is almost 10.6% of S. sonnei isolates, which is of concern.
Benzylpenicillin and ciprofloxacin resistance rates for N. gonorrhoeae remain steady at around 30%; however, resistance to azithromycin and decreased susceptibility to ceftriaxone are low but gradually increasing.
The Commission's surveillance of AMR will continue to include monitoring resistance in the community.

Read the full AURA 2016 First Australian report on antimicrobial use and resistance in human health on the Commission’s website.
References
- World Health Organization. Fact file: 10 facts about antimicrobial resistance. Fact 4. (Accessed 8 June 2016).
- Australian Commission on Safety and Quality in Health Care. AURA 2016: First Australian report on antimicrobial use and resistance in human health. Sydney: ACSQHC, 2016.
- National Centre for Antimicrobial Stewardship and the Australian Commission on Safety and Quality in Health Care. Antimicrobial prescribing and infections in Australian residential aged care facilities: Results of the 2015 Aged Care National Antimicrobial Prescribing Survey pilot. Sydney: ACSQHC, 2016.
- Smith R and Coast J. The economic burden of antimicrobial resistance: why is it more serious than current studies suggest. Technical report. London: London School of Hygiene and Tropical Medicine 2012.
- World Health Organization: Fact file: 10 facts about antimicrobial resistance. Fact 2. (Accessed 8 June 2016).
- Antibiotic Expert Group. Therapeutic guidelines: antibiotic, version 14. Melbourne: Therapeutic Guidelines Limited, 2010.
- AdrIaenssens et al. European Surveillance of Antimicrobial Consumption (ESAC). Disease-specific quality indicators for outpatient antibiotic prescribing. BMJ Qual Saf. 2011;20:764-772, [PubMed].
- NPS MedicineWise. MedicineInsight post market review report 3: antibiotics (unpublished). 2015.