Key points
- For people aged ≥ 12 years needing a medium- or high-dose inhaled corticosteroid with long-acting beta-2 agonist for asthma control
Do not use for first-line treatment of asthma. - This fixed-dose combination medicine has not been tested for, and is not indicated for, use as a reliever or a combined preventer and reliever
Patients must have a short-acting beta-2 receptor agonist reliever inhaler with them at all times. - For once daily use
Do not assume that switching from twice-daily to once-daily treatment will improve adherence — this has not been demonstrated for this product. - Available as fluticasone furoate 100 or 200 micrograms with vilanterol 25 micrograms
The inhaled corticosteroid component is equivalent in efficacy to medium- and high-dose fluticasone propionate. - Efficacy of the 100/25 microgram combination once daily is comparable to that of fluticasone propionate 250 micrograms with salmeterol 50 micrograms twice daily
The efficacy of fluticasone furoate/vilanterol for reducing incidence of flare-ups has not been compared with that of other inhaled corticosteroid/long-acting beta-2 agonist combinations in trials.
Evidence snapshot
What is known about this combination drug?
Fluticasone furoate/vilanterol is a fixed-dose combination (FDC) preventer containing the inhaled corticosteroid (ICS) fluticasone furoate and the long-acting beta-2 agonist (LABA) vilanterol. These two components are not currently available individually for the treatment of asthma.
The dose is delivered using a specific dry-powder inhaler (DPI) device, the Ellipta inhaler. The efficacy of the 100/25 microgram once-daily formulation for improving lung function is similar to that of medium-dose fluticasone propionate/salmeterol 250/50 micrograms used twice daily.
Areas of uncertainty
In people whose asthma is poorly controlled on low-dose ICS/LABA, the efficacy of fluticasone furoate/vilanterol compared with that of currently available FDCs other than fluticasone propionate/salmeterol is not known.
The adherence benefits of once-daily dosing of this FDC compared with twice-daily dosing have not been shown.
The efficacy of the higher dose has not been compared with that of other available FDCs.
It is not known how fluticasone furoate/vilanterol compares with FDCs other than fluticasone propionate/salmeterol for asthma flare-ups in the long term.
This FDC has not been tested in some high-risk groups. Safety data are limited to 52 weeks.
What does NPS MedicineWise say?
Ensure ICS/LABA FDCs are used at the correct place in therapy — for people aged ≥ 12 years needing a medium- or high-dose inhaled corticosteroid with long-acting beta-2 agonist for asthma control. Do not use for first-line treatment of asthma.
Only two strengths of fluticasone furoate/vilanterol are available; there is no low-dose* ICS option (as either monotherapy or FDC).
Follow guidelines recommending that treatment for adults whose asthma is poorly controlled on low-dose ICS* should be stepped up to low-dose ICS/LABA, then to medium-dose ICS/LABA if required.
Do not assume that switching to once-daily dosing will improve adherence. The medicine is taken once daily, which may improve adherence in some people.
However, to avoid over-treatment address poor adherence before considering any step-up in treatment. See NPS Medicinewise News Asthma – steps to control.
Consider this FDC for patients whose poor adherence cannot be improved and who may therefore benefit from once-daily dosing.
Fluticasone furoate/vilanterol is not PBS listed for use as a combined preventer and reliever; patients need to have a short-acting beta-2 agonist (SABA) reliever available at all times in case of a flare-up.
Note
The 100/25 microgram combination is also PBS listed for patients with symptomatic chronic obstructive pulmonary disease. This is a Restricted Benefit listing for patients with FEV1 < 50% predicted and repeated exacerbations with significant symptoms despite regular beta-2 agonist bronchodilator therapy. For more information on the use of LABA/ICS combination bronchodilators in COPD refer to the August 2007 RADAR review Fluticasone propionate with salmeterol xinafoate (Seretide) for chronic obstructive pulmonary disease and the December 2014 In Brief article Pharmacological therapies for chronic obstructive pulmonary disease in Australia.
* See the Australian Asthma Handbook or Therapeutic Guidelines for definitions of ICS dose levels.
See www.nps.org.au/asthma and the Australian Asthma Handbook
for further information on adherence and inhaler technique.
PBS listing
Restricted benefit
For patients who have previously experienced frequent episodes of asthma while receiving treatment with oral corticosteroids or optimal doses of inhaled corticosteroids. Patients must be aged 12 years or over.
May be prescribed by nurse practitioners (shared care model)
Authorised nurse practitioners may prescribe this medicine as part of a formal care plan with a medical practitioner. See the PBS website for more information on nurse practitioner PBS prescribing.
What is it?
Fluticasone furoate/vilanterol is an FDC delivered by DPI that combines the ICS fluticasone furoate and the LABA vilanterol.1
Fluticasone furoate is a synthetic ICS with potent anti-inflammatory activity, although its precise mechanism of action in asthma treatment is not known.
Vilanterol is a selective LABA.1-3
In patients with asthma, fluticasone furoate 100 micrograms once daily is approximately equivalent to fluticasone propionate 250 micrograms twice daily, and fluticasone furoate 200 micrograms once daily is approximately equivalent to fluticasone propionate 500 micrograms twice daily.1
Who is it for?
Fluticasone furoate/vilanterol FDC treatment is indicated for people who need medium- or high-dose ICS/LABA treatment to achieve and maintain good asthma control.1, 4
Fluticasone furoate/vilanterol 100/25 may be suitable for:
- people with asthma poorly controlled on low-dose ICS/LABA combination treatment (despite good adherence and correct inhaler technique)
- those wishing to switch from an alternative medium-dose ICS/LABA (or separate inhalers used to achieve this combination)
- those stepping down from a higher-dose ICS/LABA.
Fluticasone furoate/vilanterol 200/25 may be suitable for people whose asthma is uncontrolled by medium-dose ICS/LABA or those switching from another high-dose ICS/LABA combination.
Fluticasone furoate/vilanterol may be considered in particular for people who need medium- or high-dose ICS/LABA whose adherence is poor and cannot be improved using twice-daily dosing.
Not for use in children under 12
This FDC therapy is TGA approved and PBS listed for adults and children ≥ 12 years.
High dose not for use in patients aged 75 or older
The 200/25 dose is not recommended for use in this population because of limited data.1
Not for people with severe milk-protein allergy
This medicine contains milk protein and is contraindicated in this population.1
Where does it fit?
Do not use fluticasone furoate/vilanterol as first-line treatment
Treatment of asthma is a stepwise process that aims to achieve good asthma control by optimising the current level of symptom control and minimising the risk of future adverse outcomes.5
Guidelines highlight that most adults can achieve good asthma control with low-dose ICS if they use their inhaler correctly.5
People unable to achieve good asthma control at the current level (step) of treatment, despite demonstrated correct inhaler technique, good adherence and a confirmed diagnosis, should increase (step up) their medicine intensity to achieve good asthma control (Figure 1).5
Some people may need to be stepped up to low-dose ICS/LABA to achieve good asthma control, and few people will need to be stepped up to a higher-dose ICS/LABA (Figure 1).6
Figure 1. Stepwise treatment of asthma in adultsa
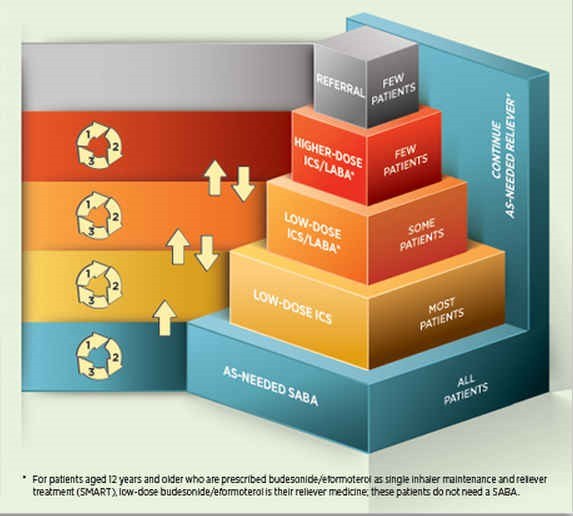
The two available strengths of fluticasone furoate (100 and 200 micrograms [used once daily]) produce similar effects to those achieved with medium- and high-strength fluticasone propionate, respectively, used twice daily.1
The Australian Asthma Handbook defines good asthma control in adults as:5
- daytime symptoms twice a week or less
- need for reliever twice a week or less†
- no limitation of activities
- no symptoms during the night or on waking.
† Not including SABA taken prophylactically before exercise.
Fluticasone furoate/vilanterol FDC is one treatment option
Other FDCs available on the PBS for the treatment of asthma are fluticasone propionate/eformoterol (Flutiform), budesonide/eformoterol (Symbicort) and fluticasone/salmeterol (Seretide).
Not approved for use as a reliever
Fluticasone furoate/vilanterol is indicated for use as a combined maintenance/preventer medication. This FDC therapy is neither TGA approved nor PBS listed for use as a reliever, and people using it also need to have a SABA reliever inhaler with them at all times.
Fluticasone furoate/vilanterol is not approved for use as single maintenance and reliever therapy (SMART) and was not tested as such in the key trials, where salbutamol was used as a reliever.6, 7
High dose not PBS listed for the treatment of COPD
The 200/25 formulation of fluticasone furoate/vilanterol is neither TGA approved nor PBS listed for the treatment of COPD.1, 4 The 100/25 formulation is PBS listed for the treatment of COPD.1, 4
Not tested in some high-risk groups
Efficacy and safety of fluticasone furoate/vilanterol has not been tested in people with a history of life-threatening asthma in the last 5 years or a flare-up requiring oral corticosteroids within the last 12 weeks.6, 8, 9
Some of the key asthma studies also excluded people who had used tobacco products in the 3 months before screening or with a historical use of 10 or more pack–years,9 or current smokers with a history of 10 pack–years‡.8
‡ Calculated by multiplying the number of packs of cigarettes smoked per day by the number of years the person has smoked, eg, 1 pack–year is equal to smoking one pack per day for 1 year, or 2 packs per day for half a year, etc.10
Educating and training patients is important with all asthma treatments
Educate patients to detect and manage deteriorating asthma, and in the optimal use of their medicine, by providing them with information about asthma and the importance of self-monitoring.5
Instruct patients in the proper use and care of the inhaler and assess their inhaler technique before starting treatment and at every consultation thereafter.5 Correct use of the inhaler is essential for successful treatment.
See www.nps.org.au/asthma for checklists to assist with inhaler technique training.
Advise patients to read the Consumer Medicine Information (CMI) leaflet.
Provide patients with a written asthma action plan
Self-management plans that include a written asthma action plan11 improve outcomes, including unscheduled doctor visits12 and hospitalisations12, 13 for asthma.
Select a written asthma action plan appropriate for the person’s age, educational status, language and culture.14
Review the written asthma action plan every year in adults and whenever there is a significant change in the patient’s treatment or asthma status.5
A range of different written asthma plans is available, including the National Asthma Council of Australia Asthma Action Plan.14-17
Review asthma control regularly
Review at least every 6–12 months in people whose asthma is well controlled, and every 3 months for high-risk patients.
Also review asthma control after an asthma flare-up, opportunistically at non-asthma visits and when a person presents with uncontrolled asthma symptoms.
Review adults 1–3 months after treatment is started/adjusted or earlier in those with very poorly controlled asthma at presentation. Review adults every 4–6 weeks during pregnancy.5
See the Australian Asthma Handbook for further details on reviewing asthma treatment and risk factors for poor outcomes.5
How does it compare?
The efficacy of fluticasone furoate/vilanterol FDC was assessed in two key randomised controlled trials.6, 7
Fluticasone furoate/vilanterol (100/25) used once daily has been directly compared with fluticasone propionate/salmeterol (250/50) used twice daily.7
The efficacy of fluticasone furoate/vilanterol (200/25) used once daily has been directly compared with fluticasone furoate monotherapy (200 microgram) used once daily§ and fluticasone propionate monotherapy (500 microgram) used twice daily.6
§ Fluticasone furoate monotherapy is currently not marketed for the treatment of asthma. Studies showing the efficacy of the monotherapy may not reflect the behaviour of the FDC.
Fluticasone furoate/vilanterol and fluticasone propionate/salmeterol have similar efficacy
Improvement in lung function in people using fluticasone furoate/vilanterol 100/25 once daily is similar to that in people using fluticasone propionate/salmeterol (250/50) twice daily (Table 1).7
This was shown in a 24-week, randomised, double-blind double-dummy study (n = 806). Trial participants were aged ≥ 12 years, were symptomatic and were considered to have poorly controlled asthma at baseline.7
Table 1 Improvement in lung function|| at 24 weeks based on analysis of the intention-to-treat trial population7
|
Intervention |
Change from baseline in 0–24-hour serial weighted mean FEV1 at 24 weeks |
|---|---|
|
FF/VI (100/25) once daily |
0.341 L |
|
FP/SAL(250/50) twice daily |
0.377 L |
|
FF/VI vs FP/SAL¶ |
–0.037 L (95% CI –0.088L to – 0.015L) p = 0.162 |
|| From baseline in 0–24-hour serial weighted mean FEV1
¶ Adjusted mean treatment difference
FF: fluticasone furoate, VI: vilanterol, FP: fluticasone propionate, SAL: salmeterol, CI: confidence interval
The minimally important difference for improvement or worsening of FEV1, based on patient perception of change, is about 10%. An improvement of ≥ 12% or 0.2 L is usually considered to be significant according to the Official American Society/European Respiratory Society statement on asthma control and endpoints.18
Trial participants were previously using a stable, medium-dose ICS (defined as fluticasone propionate 250 microgram twice daily, or equivalent) for ≥ 4 weeks, with or without LABA.
At baseline, around 70% of trial participants in each treatment arm were using ICS with a LABA. All trial participants were switched to 250 microgram fluticasone propionate twice daily for ≥ 4 weeks pre-baseline.7
Similar improvements (from baseline to week 24) were reported in the Asthma Control Test in patients using fluticasone furoate/vilanterol (mean change 2.3 ± 0.16 from 18.9 at baseline) or fluticasone propionate/salmeterol (mean change 2.0 ± 0.16 from 18.8 at baseline).7
Quality-of-life improvements, as measured using the Asthma Quality of Life + 12 Questionnaire (AQLQ+12), were also similar between the two arms (mean change 0.46 ± 0.043 from 5.35 at baseline for fluticasone furoate/vilanterol, and 0.37 ± 0.043 from 5.37 at baseline for fluticasone propionate/salmeterol).7
However, the clinical significance of these improvements is not clear, as the improvement in ACLQ+12 is below the quoted minimal important difference of 0.5 for AQLQ+12,18, 19 and the improvement in Asthma Control Test for both treatments is close to the cut-off of 1.88, corresponding to a change of one level in a physician rating of asthma control.18
Fluticasone furoate 100 micrograms once daily is similar in efficacy to fluticasone propionate 250 micrograms twice daily
In a randomised controlled trial, both treatments statistically significantly increased pre-dose FEV1 compared with placebo.
By indirect comparison, the magnitude of the increase was similar for fluticasone furoate compared with fluticasone propionate; however, no formal analysis of the differences between these two treatments was planned in the study.20
Fluticasone furoate 200 micrograms once daily is non-inferior to fluticasone propionate 500 micrograms twice daily
In the intention-to-treat trial populations of a double-blind, double-dummy, randomised controlled trial, fluticasone furoate (200 micrograms once daily) was non-inferior** to fluticasone propionate (500 micrograms twice daily) with respect to pre-dose FEV1 improvement at 24 weeks.6
As noted, the efficacy of fluticasone furoate as monotherapy may not reflect its performance as an FDC ingredient.
Trial participants were aged ≥ 12 years, with asthma poorly controlled on high-dose ICS (around 25% of trial participants pre-study) or medium-dose ICS/LABA (around 75% of trial participants pre-study).
Trial participants were maintained on ICS alone during a 4-week run-in period.6
** Non-inferiority was demonstrated because the lower limit of the 95% CI was greater than the pre-defined limit of –0.125 L.
Improved adherence with once-daily dosing not demonstrated
Once-daily dosing can improve adherence compared with twice-daily dosing.21, 22 However, adherence is influenced by factors other than just the complexity of the dosing, such as shared decision making and the direction of treatment adjustment (step up or step down).21, 23
In the key trial,7 adherence was similar for fluticasone furoate/vilanterol 100/25 used once daily compared with fluticasone propionate/salmeterol 250/50 used twice daily (> 94% in both treatment arms).
However, the nature of this double-blind double-dummy trial makes it unsuitable for assessing the effect of once- versus twice-daily dosing on adherence.7
Safety issues
As with other FDC formulations it is important that people are aware which of their current medicines are being replaced and that they stop using these medicines. People using fluticasone furoate/vilanterol also need to have a SABA reliever with them at all times.
Show patients which medicines are being replaced and advise them to return unneeded medicines to a pharmacy for safe disposal. This will reduce the risk of medicine errors. A Home Medicines Review may also be helpful for some patients.24
Effect on preventing flare-ups unclear compared with other FDCs
Incidence of flare-ups was similar with fluticasone furoate/vilanterol 100/25 used once daily compared with fluticasone propionate/salmeterol 250/50 used twice daily in the key efficacy study. However, at 24 weeks’ duration this study may be too short to assess efficacy in terms of preventing flare-ups.7
Similar adverse-effect profile to that of fluticasone propionate/salmeterol
In the key study,7 incidence of adverse events was similar with fluticasone furoate/vilanterol 100/25 used once daily and fluticasone propionate/salmeterol 250/50 used twice daily.7
Nasopharyngitis and headache are common adverse events
Combined data from trials using fluticasone furoate/vilanterol in the treatment of asthma indicate that nasopharyngitis and headache are very common adverse events associated with this treatment (reported by more than one in 10 people).1
The Product Information details a number of additional common adverse events (reported by at least three in 100 people), including URTI, bronchitis, influenza, oral candidiasis, oral pharyngeal pain and sinusitis.1
In the key efficacy trial,7 nasopharyngitis was reported by 11% of trial participants in each study arm, and headache was reported by 8% taking fluticasone furoate/vilanterol and 10% taking fluticasone propionate/salmeterol.7
Similar incidence of flare-ups to that of fluticasone propionate
The safety of fluticasone furoate/vilanterol 100/25 or 200/25 once daily was compared with that of fluticasone propionate 250/50 twice daily in a 52-week, double-blind, double-dummy, randomised controlled trial (n = 503).25
Trial participants were previously using medium- to high-dose ICS (500–1000 micrograms/day) with or without LABA. The incidence of adverse events, including severe flare-ups,†† was similar between treatment arms.25
†† Defined according to the American Thoracic Society/European Respiratory Society taskforce guidelines as deterioration of asthma requiring use of systemic corticosteroids for ≥ 3 days, or an inpatient hospitalisation or emergency room visit due to asthma that required systemic corticosteroids.
Use with caution in people with severe cardiovascular disease
Cardiovascular effects such as cardiac arrhythmias (eg, supraventricular tachycardia and extrasystoles) may be seen with sympathomimetic medicines.1
Good asthma control during pregnancy and breastfeeding is paramount
Optimal asthma control during pregnancy is vital for the mother and baby and a harm–benefit assessment should be undertaken for each patient.26
Fluticasone furoate/vilanterol FDC therapy has not been tested in pregnant or breastfeeding women in adequate or well-controlled studies.1 It is Australian category B3 for use in pregnancy.1
Consider switching to a budesonide-based treatment for women planning a pregnancy, as it is a category-A-rated drug.5
During pregnancy, however, to reduce the risk of a flare-up, women should continue with the same medicine they used before they became pregnant, especially if this treatment controlled their asthma.5
Review asthma control every 4–6 weeks during pregnancy.
Prolonged treatment may result in adrenal suppression and acute adrenal crisis
Be vigilant for the development of systemic adverse effects in all patients using an ICS.27
Adrenal suppression can occur with high doses of ICS.28 Consider the need for additional corticosteroids during periods of physiological stress.28 Do not stop treatment suddenly in these patients.28
As fluticasone furoate is a new ICS, more information is needed around its effect on cortisol suppression relative to other ICSs.29
In the key safety study, 24-hour urinary cortisol excretion showed little change from baseline at weeks 12, 28 and 52.25
However, trial participants may already have had suppressed adrenal function due to use of ICS before randomisation.30
The short-term effects of fluticasone furoate/vilanterol on hypothalamic–pituitary axis function were also assessed in a 6-week study that concluded that both strengths are non-inferior§§ to placebo.31
However, the outcomes of this trial cannot predict any potential inhibitory effect of fluticasone furoate/vilanterol on cortisol during prolonged use in a real-world clinical setting.31
For information about reporting adverse reactions to the TGA, or to report suspected adverse reactions online, see the TGA website or use the ‘Blue Card’ distributed with the October issue of Australian Prescriber.
§§ This study compared the weighted mean 0–24-hour serum cortisol concentration at baseline relative to day 42 of the two strengths of fluticasone furoate/vilanterol with placebo.31 Non-inferiority was demonstrated, as the lower limit of the 95% CI was greater than the pre-defined margin of 0.8.31
Reason for PBS listing
The Pharmaceutical Benefits Advisory Committee recommended the listing of fluticasone furoate/vilanterol DPI for maintenance treatment of asthma on a cost minimisation basis — that is, similar efficacy and cost — compared with fluticasone propionate/salmeterol (DPI and metered-dose inhaler).
The PBAC agreed that the restriction for fluticasone furoate with vilanterol FDC should be consistent with the restrictions for the other ICS/LABA FDCs listed for asthma maintenance therapy, but that the PBS-subsidised use of fluticasone furoate with vilanterol FDC should be additionally restricted to patients aged ≥ 12 years.
The PBAC noted safety concerns given that:
- neither component of the FDC is available as a single product
- there are limited long-term safety data for vilanterol
- cardiovascular concerns have been raised in regard to very-long-acting LABAs.
These concerns were weighed against the existing significant clinical experience with ICS/LABA FDC products in asthma.
Dosing issues
Fluticasone furoate/vilanterol combination therapy is available in two fixed-dose combinations:1
- fluticasone furoate 100 micrograms with vilanterol 25 micrograms
- fluticasone furoate 200 micrograms with vilanterol 25 micrograms.
Doses are taken once daily. Each inhaler contains 30 doses.
Doses in adults and children aged 12 years or over
The recommended starting dose is 100/25 micrograms for patients aged ≥ 12 years with asthma poorly controlled with low-dose ICS/LABA, or well controlled on medium-dose ICS-LABA and wishing to switch treatments.1
The higher 200/25 microgram formulation is an option for patients under 75 who cannot achieve good asthma control on the 100/25 dose or a medium-dose ICS-LABA.1
No dose adjustment is recommended for adolescents.1
The manufacturer also recommends 100/25 as a suitable starting dose for patients with asthma poorly controlled on low- or medium-dose ICS monotherapy.1 However, current Australian guidelines recommend that patients with asthma poorly controlled on low-dose ICS are first stepped up to low-dose ICS/LABA (as opposed to a medium-dose ICS/LABA such as this FDC).5
Similarly, treating with medium-dose ICS monotherapy is not a recommended or preferred treatment option.5
Ensure the patient has good adherence and can demonstrate correct inhaler technique before considering stepping up treatment.5
Note that stepping up from low-dose ICS-LABA to this FDC involves changing inhaler type, and the patient will have to become competent with a new technique.
Consider stepping down treatment if the person has good symptom control for 2–3 months, after taking into account their risk factors for flare-ups.5
Children under 12
Fluticasone furoate/vilanterol FDC is not for use in children under 12,1 as safety and efficacy have not been established in this age group.32
People aged 75 or older
The higher dose (200/25) is not recommended for people aged 75 or older because of lack of data.1
No dose adjustment in people with renal impairment
The manufacturer recommends no dose adjustment in people with renal impairment, based on the findings of a 7-day, small open-label study in people with severe renal impairment (n = 9) and healthy controls (n = 9).1, 34
Do not use the 200/25 dose in patients with hepatic impairment
Caution is advised when prescribing either dose in people with hepatic impairment.1, 33 Use only the 100/25 dose in people with moderate-to-severe hepatic impairment.1
Hepatic impairment (mild, moderate and severe) increases systemic exposure to fluticasone furoate and, in a small study using fluticasone furoate/vilanterol 200/25, moderate impairment was associated with a clinically significant reduction in serum cortisol.33
The higher dose has not been tested in people with severe hepatic impairment.33
Monitor patients with moderate to severe hepatic impairment using this FDC for systemic corticosteroid-related adverse reactions.1
Caution advised when used with some other medicines
Use caution when prescribing fluticasone furoate/vilanterol, or other medicines containing a beta-2 agonist, in people using MAOIs, tricyclic antidepressants or other drugs known to prolong the QTc interval. These drugs may increase the effect of beta-2 agonists on the cardiovascular system.1
The key efficacy trial excluded people taking potent CYP3A4 inhibitors within the previous 4 weeks;8 therefore take care when using fluticasone furoate/vilanterol with strong CYP3A4 inhibitors such as ritonavir. Concomitant use with strong CYP3A4 inhibitors will increase systemic exposure to both fluticasone furoate and vilanterol.1
Information for patients
Advise patients that:
- they should familiarise themselves with the step-by-step instructions for use of the inhaler described in the leaflet provided with the inhaler
- they should rinse their mouth with water and spit out after every dose to minimise the risk of candida yeast-like infections
- the inhaler should be stored and cleaned as advised and as directed in the CMI
- fluticasone furoate/vilanterol FDC must be used daily for optimal benefit, even when they are asymptomatic
- the inhaler must not be used more often than one inhalation (actuation) once daily
- the inhaler should be used at the same time each day; this can be either in the morning or in the evening
- fluticasone furoate/vilanterol FDC is not approved for use as a reliever, and a SABA must be accessible at all times and used if symptoms occur between doses
- an additional LABA must not be used under any circumstances; unneeded medicines replaced by this FDC should be returned to a pharmacy for safe destruction
- if patients experience serious asthma-related adverse events while using fluticasone furoate/vilanterol, they should continue treatment but seek medical advice
- if they begin to need to use their reliever (SABA) more frequently, they should return to their doctor immediately to have their treatment reviewed.
Discuss the fluticasone furoate with vilanterol (Breo Ellipta) Consumer Medicine Information leaflet with the patient.
References
- GlaxoSmithKline Australia Pty Ltd. Breo Ellipta Product Information. 2014. [Online]
- Procopiou PA, Barrett VJ, Bevan NJ, et al. Synthesis and structure-activity relationships of long-acting beta2 adrenergic receptor agonists incorporating metabolic inactivation: an antedrug approach. J Med Chem 2010;53:4522\u201330. [PubMed]
- Tan LD, Chan AL, Albertson TE. New combination treatments in the management of asthma: focus on fluticasone/vilanterol. J Asthma Allergy 2014;7:77\u201383. [PubMed]
- Therapeutic Goods Administration. Public Summary Document: Fluticasone Furoate and Vilanterol Trifenatate, Breo Ellipta, GlaxoSmithKline Australia Pty Ltd, 2014. [Online]
- National Asthma Council Australia. Australian Asthma Handbook. Melbourne: 2014. [Online]
- O'Byrne PM, Bleecker ER, Bateman ED, et al. Once-daily fluticasone furoate alone or combined with vilanterol in persistent asthma. Eur Respir J 2014;43:773\u201382. [PubMed]
- Woodcock A, Bleecker ER, Lotvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial. Chest 2013;144:1222\u20139. [PubMed]
- Woodcock A, Bleecker ER, Lotvall J, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: A randomized trial \u2013 Online Supplement. Chest 2013;144:1222\u20139. [PubMed]
- Busse WW, O'Byrne PM, Bleecker ER, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the beta2 agonist vilanterol administered once daily for 52 weeks in patients >12\u00a0years old with asthma: A randomised trial \u2013 Online Supplement. Thorax 2013;68:513\u201320. [PubMed]
- US National Institutes of Health National Cancer Institute. [Online] (accessed 13 February 2014).
- Reddel H. Rational prescribing for asthma in adults \u2013 written asthma action plans Australian Prescriber 2012;35:78\u201381. [Online]
- Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev 2003:CD001117. [PubMed]
- Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax 2004;59:94\u20139. [PubMed]
- National Asthma Council Australia. Asthma Action Plans 2013. [Online] (accessed 6 September 2013).
- Australian Government Department of Health and Ageing. Remote Indigenous Australian Asthma Action Plan. 2000. [PDF] (accessed 5 November 2013).
- Australian Government Department of Health and Ageing. My asthma plan 2006. [PDF] (accessed 5 November 2013).
- PIKA WIYA Health Service Inc. Everyday asthma action plan [PDF] (accessed 10 September 2013).
- American Thoracic Society. Asthma Control and Exacerbations: Standardizing Endpoints for Clinical Asthma Trials and Clinical Practice. 2009. [Online] (accessed 30 October 2014).
- Juniper EF, Svensson K, Mork AC, et al. Modification of the asthma quality of life questionnaire (standardised) for patients 12 years and older. Health Qual Life Outcomes 2005;3:58. [PubMed]
- Lotvall J, Bleecker ER, Busse WW, et al. Efficacy and safety of fluticasone furoate 100 mug once-daily in patients with persistent asthma: a 24-week placebo and active-controlled randomised trial. Respir Med 2014;108:41\u20139. [PubMed]
- Guest JF, Davie AM, Ruiz FJ, et al. Switching asthma patients to a once-daily inhaled steroid improves compliance and reduces healthcare costs. Prim Care Respir J 2005;14:88\u201398. [PubMed]
- Price D, Robertson A, Bullen K, et al. Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study. BMC Pulm Med 2010;10:1. [PubMed]
- Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med 2010;181:566\u201377. [PubMed]
- Australian Government Department of Health Services. Home Medicines Review (HMR). [Online] (accessed 24 September 2013).
- Busse WW, O'Byrne PM, Bleecker ER, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the beta2 agonist vilanterol administered once daily for 52 weeks in patients >12\u00a0years old with asthma: A randomised trial. Thorax 2013;68:513\u201320. [PubMed]
- Lim A Hussainy SY, Abramson MJ. Asthma drugs in pregnancy and lactation. Australian Prescriber 2013;36:150\u20133. [PubMed]
- Busse WW, Andersen L. Authors' reply to 'Safety and tolerability of fluticasone furoate/vilanterol'. Thorax 2013;68:1165\u20136. [PubMed]
- Rossi S (ed). Australian Medicines Handbook, 2013. Adelaide: Australian Medicines Handbook Pty Ltd. [Online] (accessed 22 April 2014).
- National Institute for Clinical Excellence. ESNM34 Asthma: fluticasone furoate/vilanterol (Relvar Ellipta) combination inhaler. 2014. [Online] (accessed 30 October 2014).
- Lipworth B. Systemic safety of fluticasone furoate/vilanterol combination. Thorax 2013;68:1165. [PubMed]
- Allen A, Schenkenberger I, Trivedi R, et al. Inhaled fluticasone furoate/vilanterol does not affect hypothalamic-pituitary-adrenal axis function in adolescent and adult asthma: Randomised, double-blind, placebo-controlled study. Clin Respir J 2013;7:397\u2013406. [PubMed]
- GlaxoSmithKline. Summary of product characteristics. 2014. [Online] (accessed 30 October 2014).
- Allen A, Davis A, Hardes K, et al. Influence of renal and hepatic impairment on the pharmacokinetic and pharmacodynamic properties and tolerability of fluticasone furoate and vilanterol in combination. Clin Ther 2012;34:2316\u201332. [PubMed]
