Practice Review – Rheumatoid arthritis
Australian rheumatologists and selected immunologists recently received a PBS Practice Review on prescribing for rheumatoid arthritis. Find out more about how to read your Practice Review.
Quality use of b/tsDMARDs and other medicines
Australian rheumatologists and selected immunologists recently received a PBS Practice Review titled ‘Rheumatoid arthritis: Quality use of b/tsDMARDs and other medicines’, developed by NPS MedicineWise in collaboration with the Australian Rheumatology Association (ARA).
The Practice Review is intended to help clinicians reflect on their prescribing of biological/targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs) and other medicines for rheumatoid arthritis (RA).
The information on this page may help prescribers interpret their individual PBS Practice Review data. National and aggregate state data are also included below.
Download and print a sample report
Frequently asked questions
Continuing professional development
For RACP Fellows, the time you spend using the ‘Quality use of b/tsDMARDs and other medicines’ Practice Review report to review and adjust your practice meets the requirements for CPD under Category 3 – Measuring Outcomes.
An optional template has been developed that you can complete and use as evidence for this activity.
Use this CPD Shortcut to record this activity in MyCPD. Some fields have been pre-populated for your convenience; please adjust these as necessary before saving the activity.
Aggregate state and national data
Use of csDMARDs before starting b/tsDMARD treatment
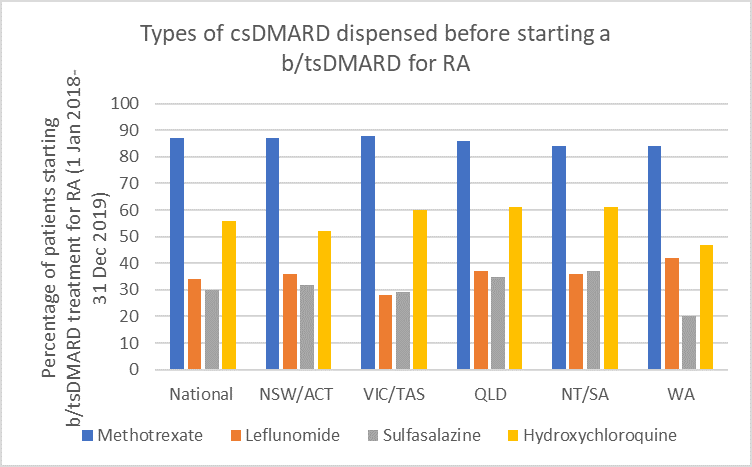
Methotrexate (MTX) remains the first-line DMARD for rheumatoid arthritis (RA).1-3 Optimise use by rapidly escalating to required dose.4,5
Types of methotrexate used before starting a b/tsDMARD for RA, 2017–2019
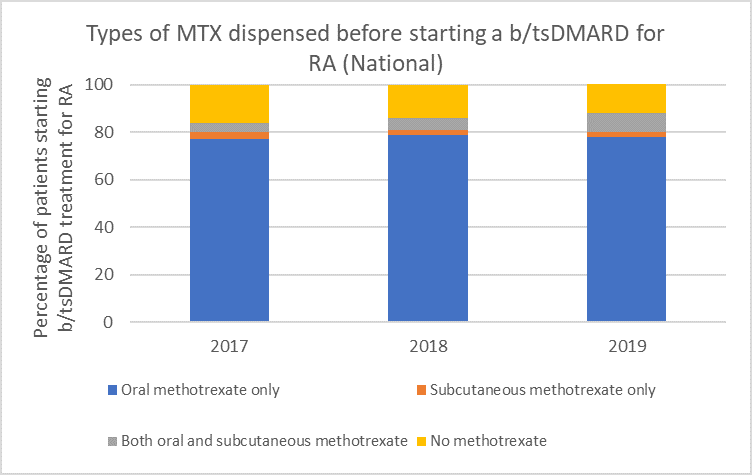
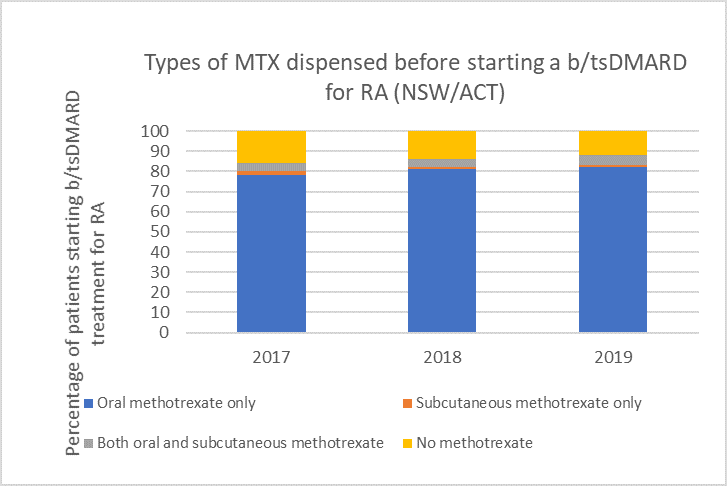
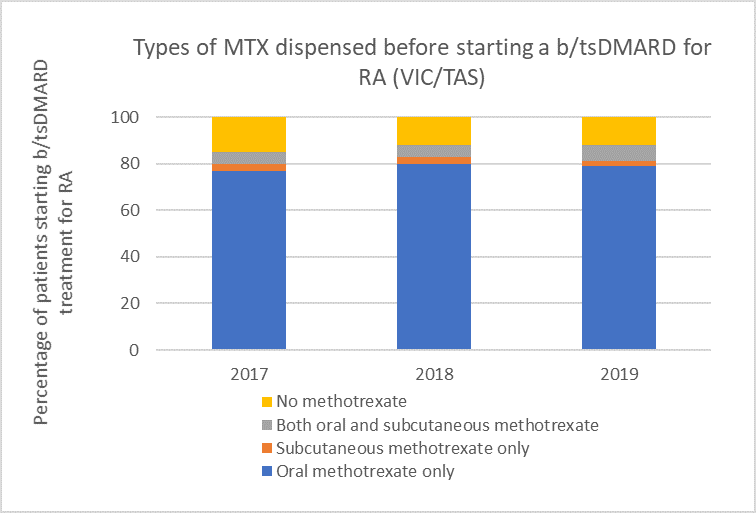
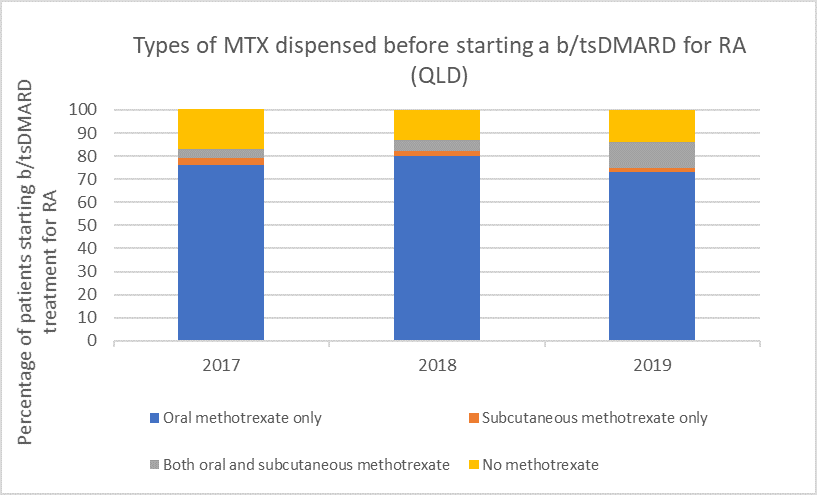
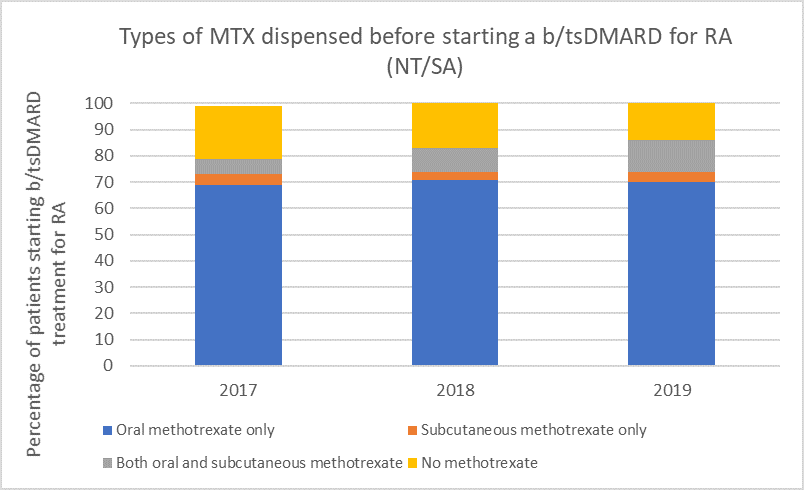
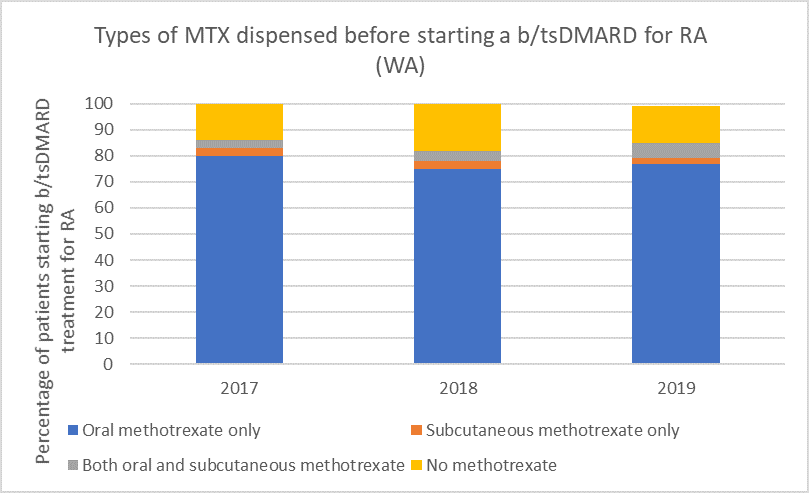
Note: Because of rounding, percentages may range from 99% to 101%.
Subcutaneous MTX is more efficacious and generally better tolerated than oral MTX, with significantly fewer associated gastrointestinal (GI) adverse effects (eg, nausea and diarrhoea).6
Switching from oral to subcutaneous MTX is highly effective; in a study 72% of patients switched remained on subcutaneous MTX for the duration of the 5-year observational period without the addition of a biologic.7
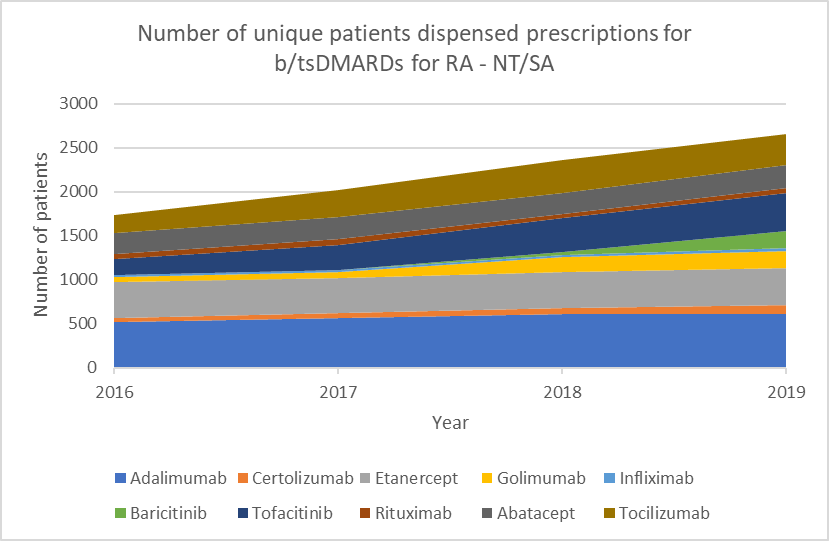
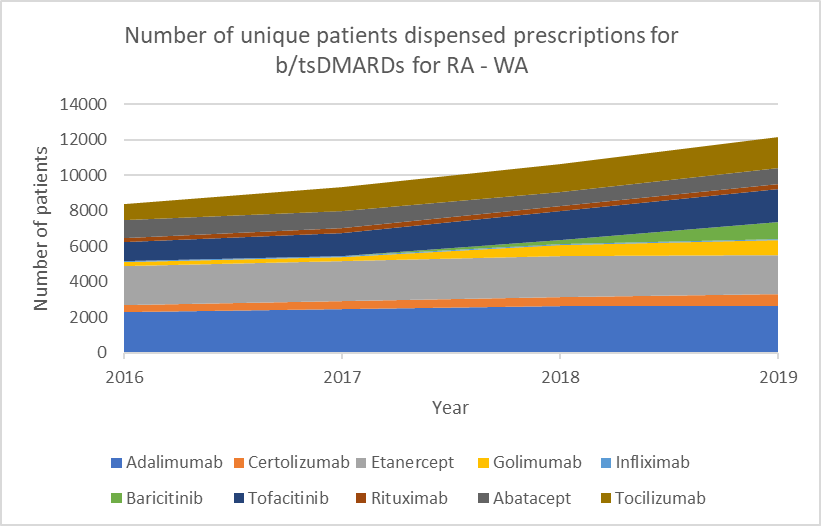
Choice of biologic when starting b/tsDMARD treatment
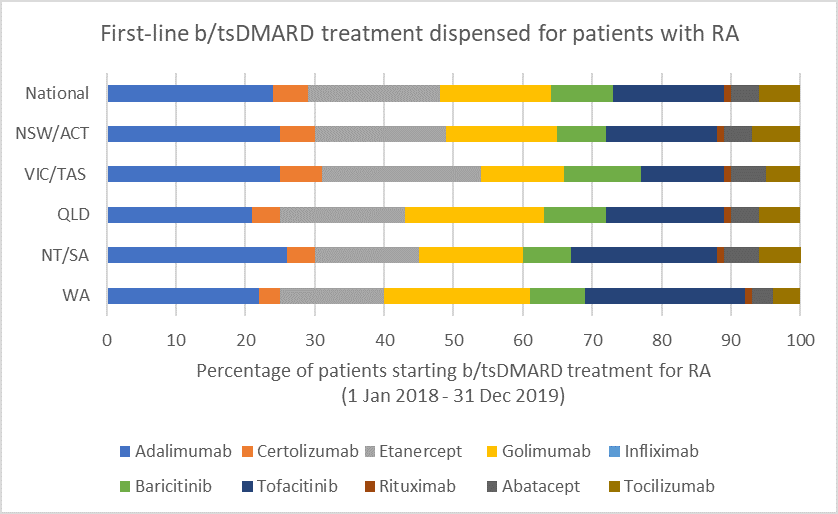
b/tsDMARDs are generally considered to have comparable efficacy for RA.1,2 Individualise choice of b/tsDMARD.
Number of unique patients dispensed prescriptions for b/tsDMARDs for RA (Australia-wide, 2016–19)
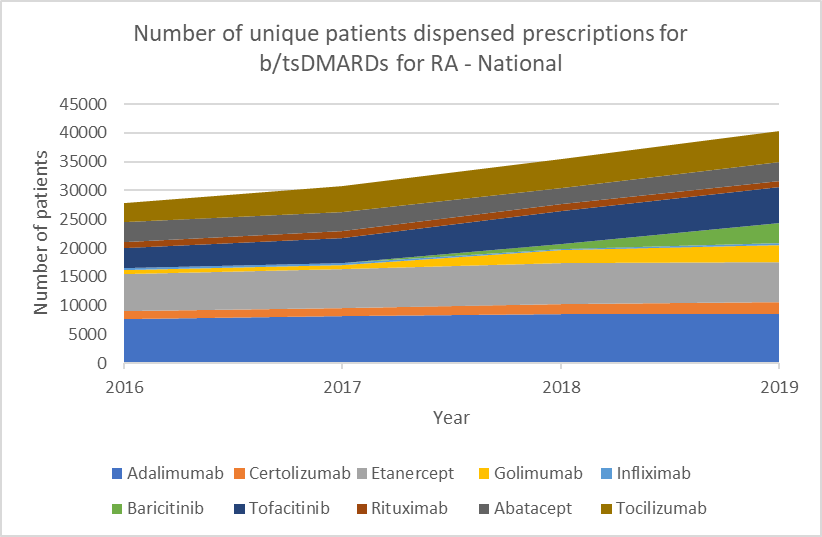
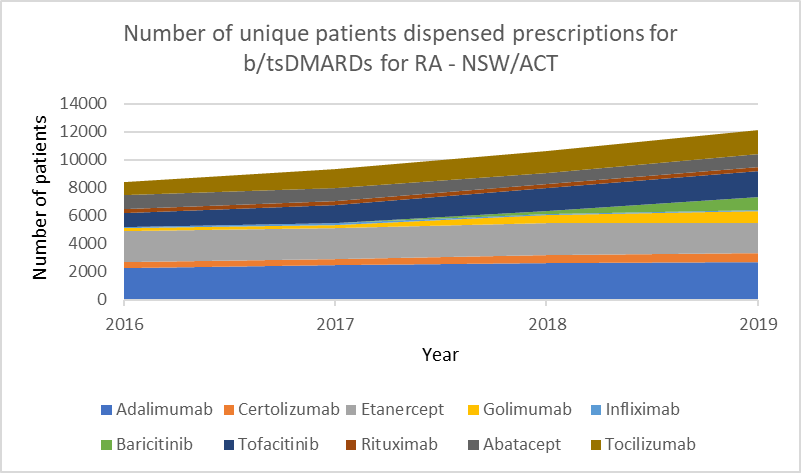
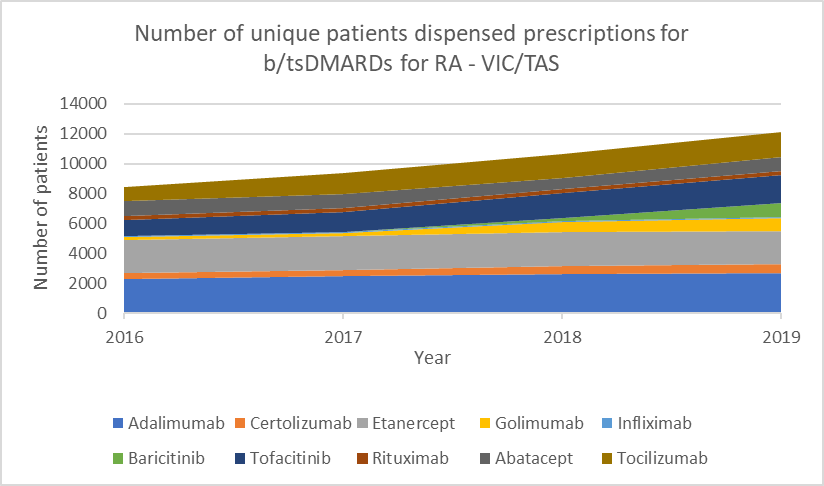
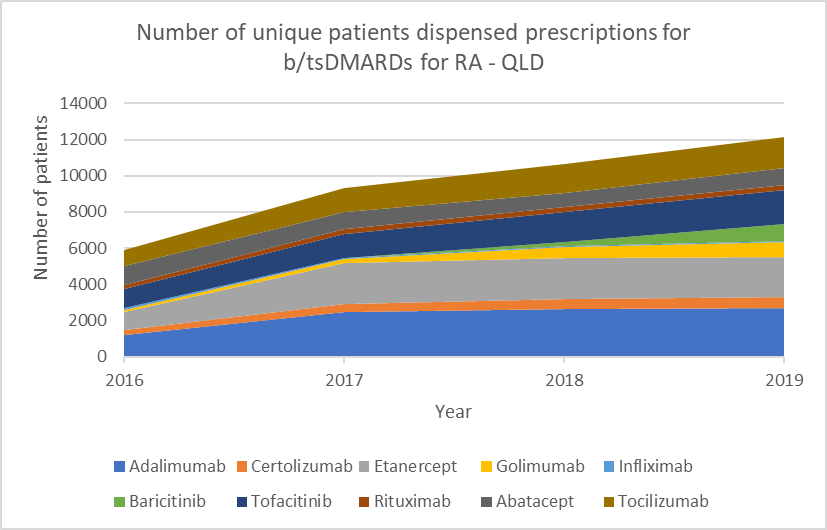
Glucocorticoid use
Between 1 November 2016 and 31 October 2018, the median prescribing rheumatologist/immunologist across Australia started b/tsDMARD treatment for RA for 12 patients. Of these 12 patients, after 6 months or more of b/tsDMARD treatment:
- 6 (44%) were dispensed prescriptions for glucocorticoids prescribed by any prescriber (including the treating rheumatologist/immunologist) during the following 12 months
- 2 (18%) were dispensed prescriptions for glucocorticoids that were prescribed by the treating rheumatologist/immunologist during the following 12 months.
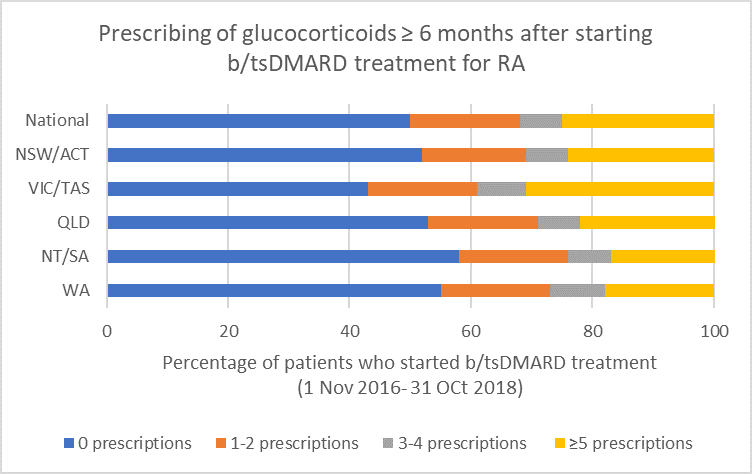
Note: Because of rounding, percentages may range from 99% to 101%.
- If a glucocorticoid is considered necessary, guidelines recommend short-term use to control flare-ups and limiting use to ≤ 3 months for bridging therapy when starting or changing DMARDs.1,2
Opioid use
Between 1 January 2018 and 31 December 2019, the median prescribing rheumatologist/immunologist across Australia had 59 patients who were prescribed b/tsDMARDs for RA. Of these, after having a b/tsDMARD prescription dispensed:
- 27 (47%) were dispensed prescriptions for opioids prescribed by any prescriber (including the treating rheumatologist/immunologist) (see Fig. 7)
- 2 (4%) were dispensed prescriptions for opioids prescribed by the treating rheumatologist/immunologist.
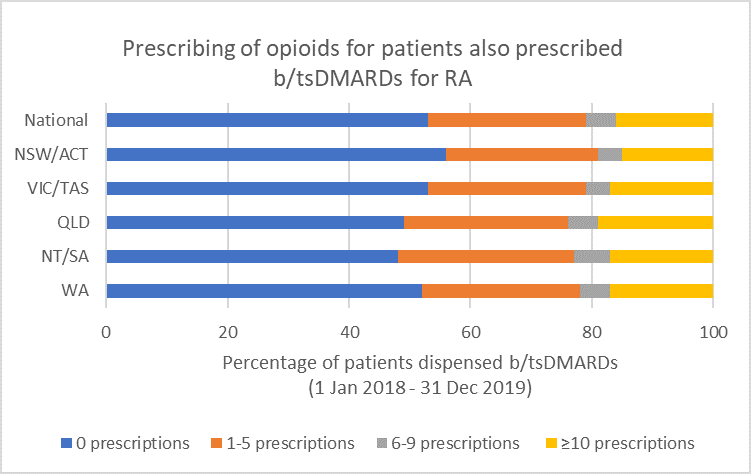
Note: Because of rounding, percentages may range from 99% to 101%.
- There is insufficient evidence to support the long-term use of opioids for pain management by patients with RA.19,20
- Any benefits of opioids can usually be determined within a 2–4 week trial of opioid treatment.16 Short-term use (< 6 weeks) of weak opioids may provide some analgesia, but adverse effects and the risk of harms (including increased risk of serious infection22 and risk of overdose) limit their use.14
Additional resources
- Webinar - Australian Living Guideline: Down-titration of b/tsDMARDs in Rheumatoid Arthritis, Axial Spondyloarthritis and Psoriatic Arthritis 4 November 2020 8 pm AEDT
Coming soon
- b/tsDMARDs down-titration algorithm
- Patient decision aid on down-titration
- Patient down-titration fact sheet
- Patient journey factsheets
- Low-dose methotrexate position statement
Find further information on additional resources and upcoming interventions
Acknowledgments and contributions
- This PBS Practice Review has been developed in collaboration with ARA representatives Prof Catherine Hill, Dr Claire Barrett and Dr Robert Baume, together with members of the Targeted Therapies Alliance (ARA, Cochrane MSK, NPS MedicineWise and UniSA).
- We are also grateful to Dr David Liew, Dr Daman Langguth, Dr Ingrid Hutton and Dr Jenny Walker for their contributions and feedback as part of user testing.

Practice Review references
- Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020.
- Therapeutic Guidelines. Melbourne: Therapeutic Guidelines Limited (accessed 26 June 2020).
- Singh JA, Saag KG, Bridges SL, Jr., et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2016;68:1-26.
- Visser K, van der Heijde D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann Rheum Dis 2009;68:1094-9.
- Mouterde G, Baillet A, Gaujoux-Viala C, et al. Optimizing methotrexate therapy in rheumatoid arthritis: a systematic literature review. Joint Bone Spine 2011;78:587-92.
- Bianchi G, Caporali R, Todoerti M, et al. Methotrexate and rheumatoid arthritis: current evidence regarding subcutaneous versus rral routes of administration. Adv Ther 2016;33:369-78.
- Rohr MK, Mikuls TR, Cohen SB, et al. Underuse of methotrexate in the treatment of rheumatoid arthritis: a national analysis of prescribing practices in the US. Arthritis Care Res (Hoboken) 2017;69:794-800.
- Pharmaceutical Benefits Scheme. PBS Schedule: Summary of Changes (April 2018). Canberra: Australian Government Department of Health, 2018 (accessed 23 June 2020).
- Australian Medicines Handbook. Folic acid. Adelaide: AMH Pty Ltd, 2020 (accessed 24 June 2020).
- Rein P, Mueller RB. Treatment with biologicals in rheumatoid arthritis: an overview. Rheumatol Ther 2017;4:247-61.
- ANZMUSC. An Australian Living Guideline for the pharmacological management of inflammatory arthritis v0.3. 2020.
- Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology (Oxford) 2019;58:e3-e42.
- Lopez-Olivo MA, Colmegna I, Karpes Matusevich AR, et al. Systematic review of recommendations on the use of disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and cancer. Arthritis Care Res (Hoboken) 2020;72:309-18.
- Isaacs JD, Cohen SB, Emery P, et al. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: a meta-analysis. Ann Rheum Dis 2013;72:329-36.
- Gottenberg JE, Courvoisier DS, Hernandez MV, et al. Brief report: association of rheumatoid factor and anti-citrullinated protein antibody positivity with better effectiveness of abatacept: results from the pan-european registry analysis. Arthritis Rheumatol 2016;68:1346-52.
- Götestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann Rheum Dis 2016;75:795-810.
- Flint J, Panchal S, Hurrell A, et al. BSR and BHPR guideline on prescribing drugs in pregnancy and breastfeeding-Part I: standard and biologic disease modifying anti-rheumatic drugs and corticosteroids. Rheumatology (Oxford) 2016;55:1693-7.
- Strehl C, Bijlsma JW, de Wit M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis 2016;75:952-7.
- Whittle SL, Richards BL, Husni E, et al. Opioid therapy for treating rheumatoid arthritis pain. Cochrane Database Syst Rev 2011:Cd003113.
- Day AL, Curtis JR. Opioid use in rheumatoid arthritis: trends, efficacy, safety, and best practices. Curr Opin Rheumatol 2019;31:264-70.
- Royal Australian College of General Practitioners. Prescribing drugs of dependence in general practice, Part C2: The role of opioids in pain management. East Melbourne: RACGP, 2017. (accessed 24 June 2020).
- Wiese AD, Griffin MR, Stein CM, et al. Opioid analgesics and the risk of serious infections among patients with rheumatoid arthritis: a self-controlled case series study. Arthritis Rheumatol 2016;68:323-31.
